Unisil Ltd.
Innovative and Implementation Company
Innovative and Implementation Company
Due to the huge number of combinations, both in terms of the nature of substituents and silsesquioxane substrates, we also offer a custom synthesis of silsesquioxane products.

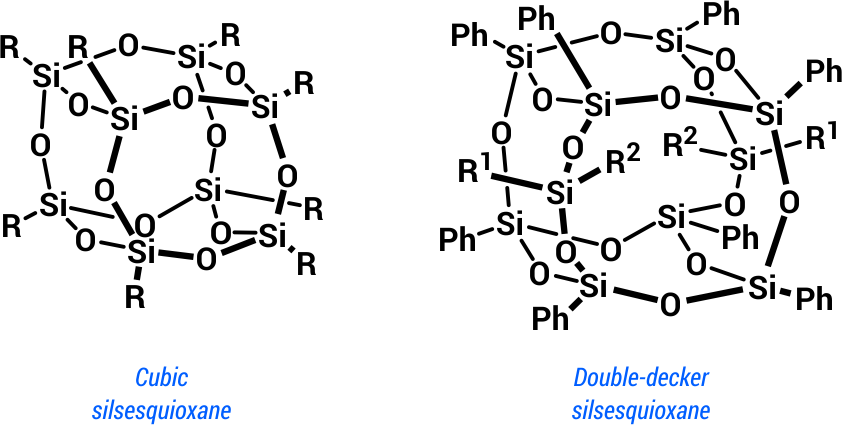
Silsesquioxanes belong to a large and diverse class of cage organosilicon hybridcompounds of the general formula [RSiO1.5]n (where n = 6, 8, 10, 12). The most studied and widely usedgroup is that of cubic octasubstituted silsesquioxanes (n = 8). Recent years saw also theemergence of a new subclass of silsesquioxanes, namely double-decker silsesquioxanes (Scheme 1).
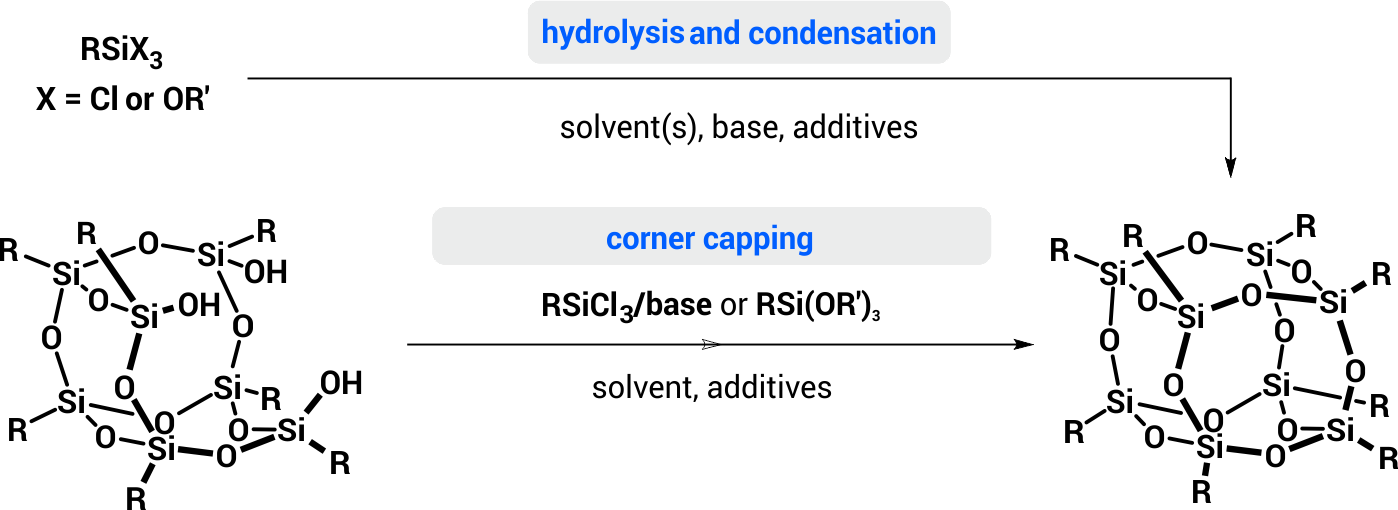
The overwhelming majority of silsesquioxane structures is prepared by condensation of the respective chloro- or alkoxysilanes, for example, fully condensed cubic silsesquixanes can be obtained via hydrolysis/condensation of trichloro- or trialkoxysilanes or by corner-capping reactions of silsesquioxane trisilanols (Scheme 2).
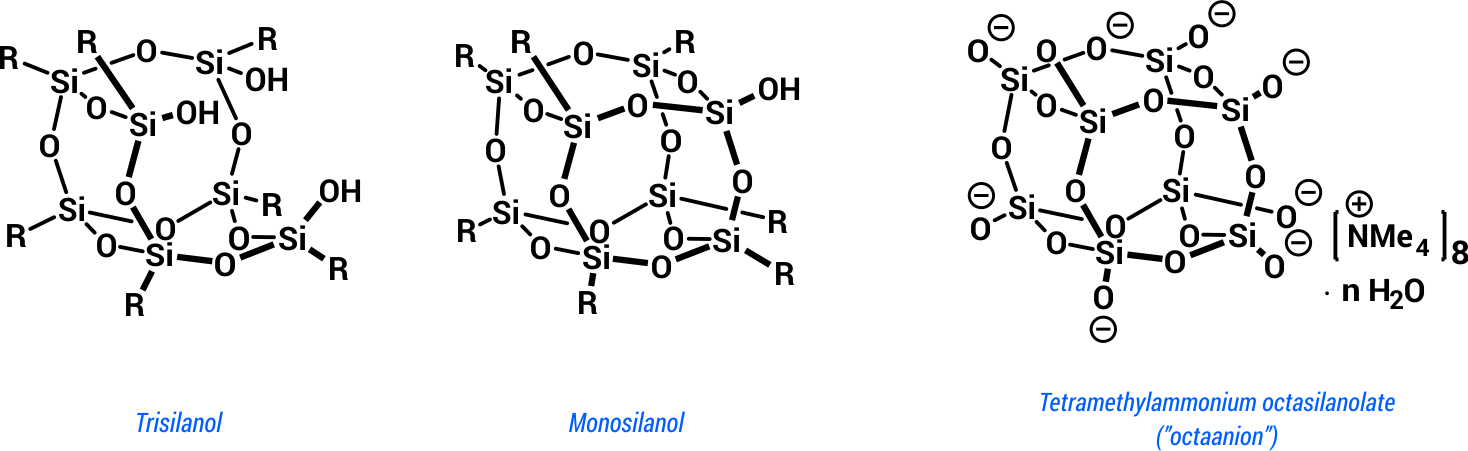
Silsesquioxane silanols or silanolates may form incompletely condensed cages as well as closed structures with the reactive silanol grups number ranging from one to eight (Scheme 3).
Silsesquioxanes are hybrid compounds that combine features of organic and inorganic systems (Scheme 4). The inorganic fragment is a rigid, chemically robust Si-O-Si core, which is the backbone of the entire structure, while the organic fragments are usually organic substituents (alkyl, aryl, alkenyl groups, etc.) attached directly to silicon atoms located in the core. These substituents may be also reactive groups (e.g., Cl, OH) that allow further direct functionalization.
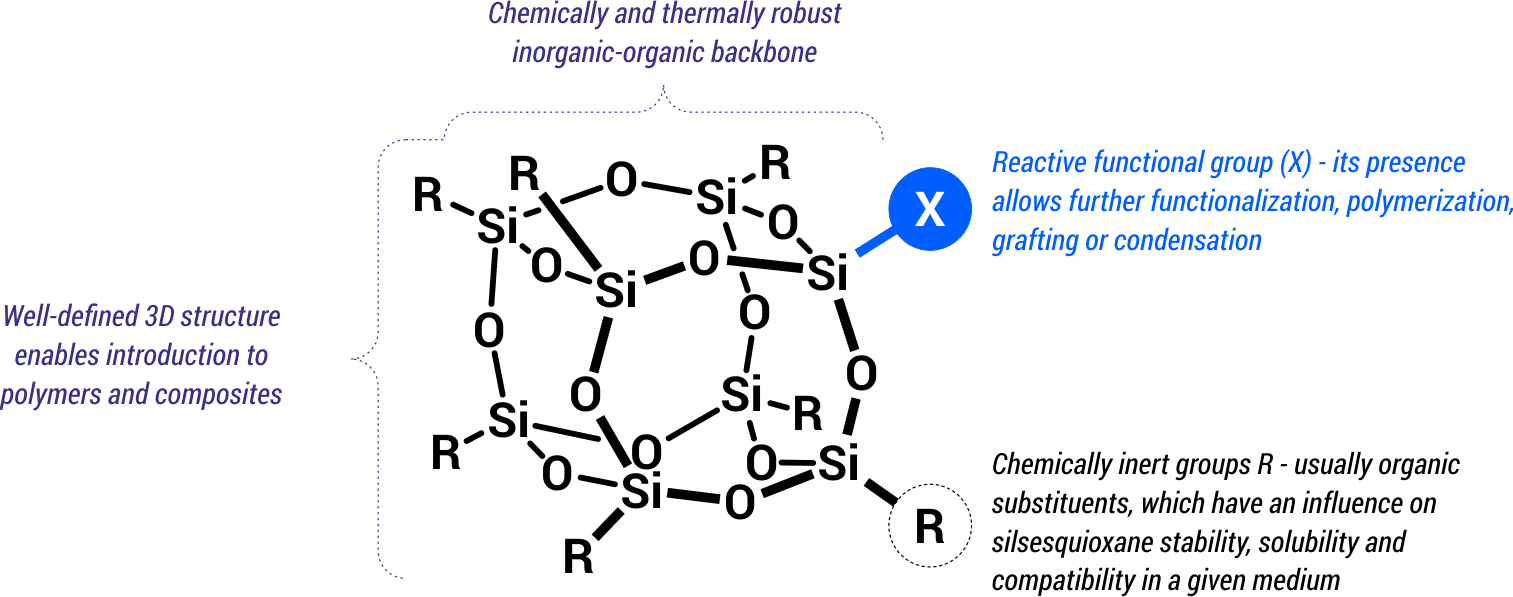
The presence of Si-O-Si groups in the silsesquioxane skeleton ensures high thermal and chemical stability of the entire system, while organic substituents affect such parameters as solubility, density, physical state and compatibility in polymer and composite systems. In turn, the presence of reactive substituents allows for the modification of silsesquioxanes by the formation of chemical bonds with new organic and organometallic functions, or by grafting with polymeric materials. The whole silsesquioxane cage forms a precisely defined, three-dimensional structure with nanometric dimensions.
The precisely defined size of silsesquioxanes, as well as the ability to create covalent bonds and non-covalent interactions with polymers make them highly effective nanofillers. Silsesquioxanes affect many properties of polymer and composite materials, among others extending the working temperature of the polymer, increasing resistance to oxidation, increasing fire resistance, raising the temperature of decomposition and vitrification and lowering the viscosity. This is related not only to their nanometric dimensions, but also to the presence of organofunctional substituents that are connected to the silicon-oxygen skeleton.
| Catalog number | |||
|---|---|---|---|
SC-TRISILANOL-001 |
1,3,5,7,9,11,14-heptaisobutyl-2,4,6,8,10,12,13,15,16-nonaoxa-1,3,5,7,9,11,14-heptasilatricyclo[7.3.3.15,11]hexadecane-3,7,14-triol |